Drug Discovery Services
Computer-Aided Molecular Design Core Facility
Director CAMD: Professor Olaf Wiest, camd@nd.edu
The Notre Dame Computer-Aided Molecular Design (CAMD) Core Facility aims to provide a full range of computational support, from atomistic modeling to assistance in proposal writing, for drug discovery and related areas to all groups on campus. For use of our computational services or to request a quote for inclusion in a grant submission, please contact us at camd@nd.edu.
Chemical Synthesis and Drug Discovery Core Facility
Director: Professor Brandon Ashfeld, bashfeld@nd.edu
The facility supports translational biomedical research by providing expertise that enables the preparation of small molecules for use in hit verification, lead development, and midsize scale‐up. In addition, the core supports the preparation of biological probes (affinity, fluorescently tagged, etc.), active pharmaceutical agents as experimental controls, and small chemical libraries for structure-activity relationships and the optimization pharmacological properties. For use of our chemical synthesis services or to request a quote for inclusion in a grant submission, please contact wrcadmin@nd.edu . List of services please click CSDD Services.
This shared user research core is also charged with organizational oversight of the products of past, current, and future chemical synthesis to create a university compound collection with currently 20,000 unique chemical entities. The establishment of appropriate intellectual property controls allows for participation of additional academic and industrial partners and the creation of a regional drug discovery consortium through the Indiana Clinical and Translational Science Institute (Indiana CTSI).
Biological Screening and Development Core Facility
Managing Director: Professor Aktar Ali, aali4@nd.edu
The Biological Screening and Development (BSD) Core provides access to equipment and expertise for the biological and translational assessment of chemical compounds. The core offers many services to the Notre Dame research community; including consulting, design, development, miniaturization, and implementation of high-throughput assays to your research needs. Additionally, the core can provide expertise and technologies to assist with and perform in vivoPK, PD and bio-distribution studies for lead compound development. The core is capable of generating and purifying recombinant proteins for numerous in vitroassays. And we offer a full range of ADME-T services for small molecule development using high-throughput pre-clinical screenings; these assays include: Microsomal Stability, Primary Hepatocyte Toxicity, Caco-2 Permeability and Transport, Plasma Stability, Plasma protein binding, hERG Interaction, AMES Mutagenicity Test, Blood-Brain Barrier Permeability, CYP Inhibition, and CYP Induction.
For use of our screening or assay development services or to request a quote for inclusion in a grant submission, please contact bsdc@nd.edu
External Drug Discovery Services and Partnerships
The CSDD Facility staff are happy to assist in the use of any of these services.
Community for Open Antimicrobial Drug Discovery

The Community for Open Antimicrobial Drug Discovery (CO-ADD) is a Wellcome Trust funded, non-for-profit, open framework for discovery and development of new therapies against multi drug-resistant (MDR) bacteria based at The University of Queensland in Australia. CO-ADD will perform primary antibacterial screening for academic research groups as a free service and makes no claims on results or IP.
In the primary screening they test against five key ESKAPE pathogens, E. coli, K.pneumonia, A.baumannii, P.aeruginosa, S.aureus (MRSA) and 2 fungi (C. neoformans and C. albicans). Hits will be further analyzed with specific panels that include MDR, Pan-resistant bacterial strains and clinical isolates as well as general cytotoxicity. Only requires 1 mg and the provider of the compound does not need to provide a structure. The Wellcome Trust asks that after 18 months, the participant should make the results available to the community (CO-ADD or chEMBL database).
For more information read about the CO-ADD in ACS Chemical Biology.
Eli Lilly's Open Innovation Drug Discovery Screening Program
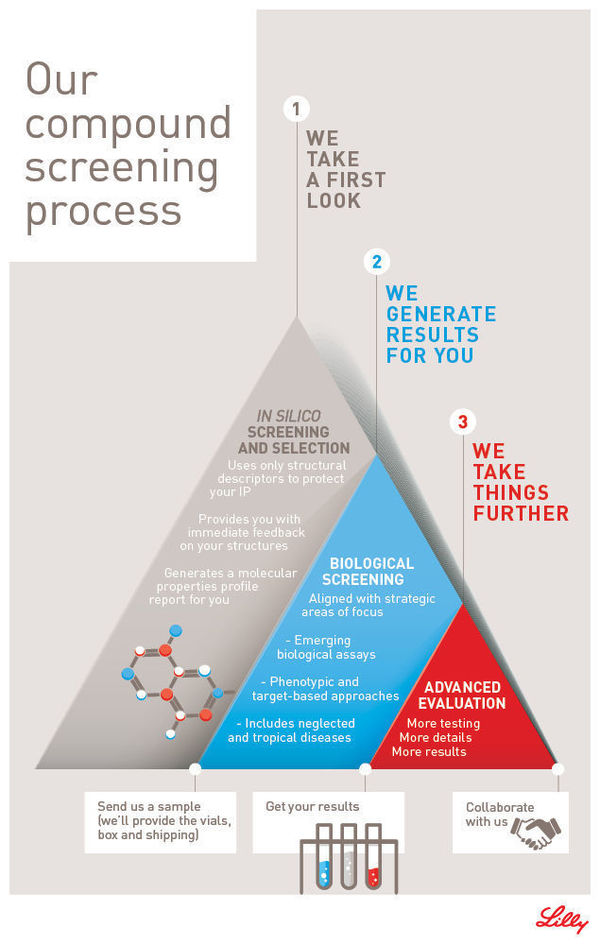
The Open Innovation Drug Discovery Screening program offering enables scientists to submit compounds for experimental evaluation in Lilly's proprietary biological assays. Results from the assays inform next steps towards further collaborative work. The OIDD screening process starts once an investigator submits compound structures to Lilly through the OIDD platform. An automated in silico analysis determines whether the compounds are eligible for biological evaluation. If that is the case, the OIDD Program provides vials and shipping as an in-kind service to participants. The entire process is run in a structure-blinded fashion, where only the results of the in silico analysis, and not the actual chemical structures, become available to Lilly.
Once Lilly receives the physical samples, they are routed for biological evaluation in several different assay modules. All biological results are provided to the investigator through their personal OIDD account. The OIDD team may choose to reach out to investigators to discuss next steps for specific compounds with a desirable profile.
Eli Lilly's Open Innovation Drug Discovery Synthesis Program
The OIDD Synthesis program offers participating investigators the opportunity to synthesize compounds remotely in Lilly's Automated Synthesis Laboratory (ASL). The ASL provides synthetic chemists the ability to explore novel synthetic approaches, improve reaction efficiency, and test the feasibility of synthetic routes to maximize the yield of targeted compounds.
CSDD staff is here to help Notre Dame researchers utilize Lilly's atomated synthesis lab to prepare unique compounds and explore interesting new chemistry contact us for assistance.
In Vitro ADME Services through Quintiles

The ADME department within Quintiles Bioanalytical and ADME Labs was created in 2011 following a decision by Eli Lilly and Co. to divest their ADME laboratory capabilities. We are now a contract research site of roughly 50 employees working in 22,000 square feet of new lab space. Our group is focused on in vitro ADME studies, including permeability, metabolic stability, metabolite identification, and drug–drug interaction (DDI) risk assessment, ranging from highly automated discovery screening platforms to definitive development studies that enable regulatory submission.
Our scientists routinely conduct industry-standard in vitro metabolism and DDI-based assays, including highly automated ADME in vitro screens. Let us help you drive your discovery-phase structure–activity relationship (SAR) by optimizing for ADME drugability properties, in parallel to your receptor binding potency and selectivity, for more rapid identification of high-quality drug candidates.
ADME Screening Services
- MDCK permeability
- Microsomal clearance (rat and human)
- Hepatocyte clearance (rat and human)
- Cytochrome P450 inhibition, single concentration
- CYP3A time-dependent inhibition, single concentration
- Plasma protein binding
- Metabolite Profiling Services
- Discovery in vitro hot-spot analysis (microsomes or hepatocytes)
- Late Phase ADME Services
MTA-based Compound Requests to Pharmaceutical Companies and the NCI Formulary.
Pharmaceutcial companies such as Johnson & Johnson, Bristol-Myers Squibb, Novartis, and Eli Lilly will consider compound requests for non-clinical research from all therapeutic areas. In addition, The National Cancer Institute (NCI) agent formulary (NCI Formulary) is a public-private partnership between the NCI and pharmaceutical and biotechnology companies with a purpose of providing academic investigators with rapid access to agents or combinations of agents for cancer clinical trial use as well as pre-clinical research; particularly, experiments focused on agents targeting molecular pathways from multiple collaborating pharmaceutical companies.
The Warren Center is happy to assist Notre Dame researchers with MTA submissions and compound management. Please contact the CSDD for assistance.